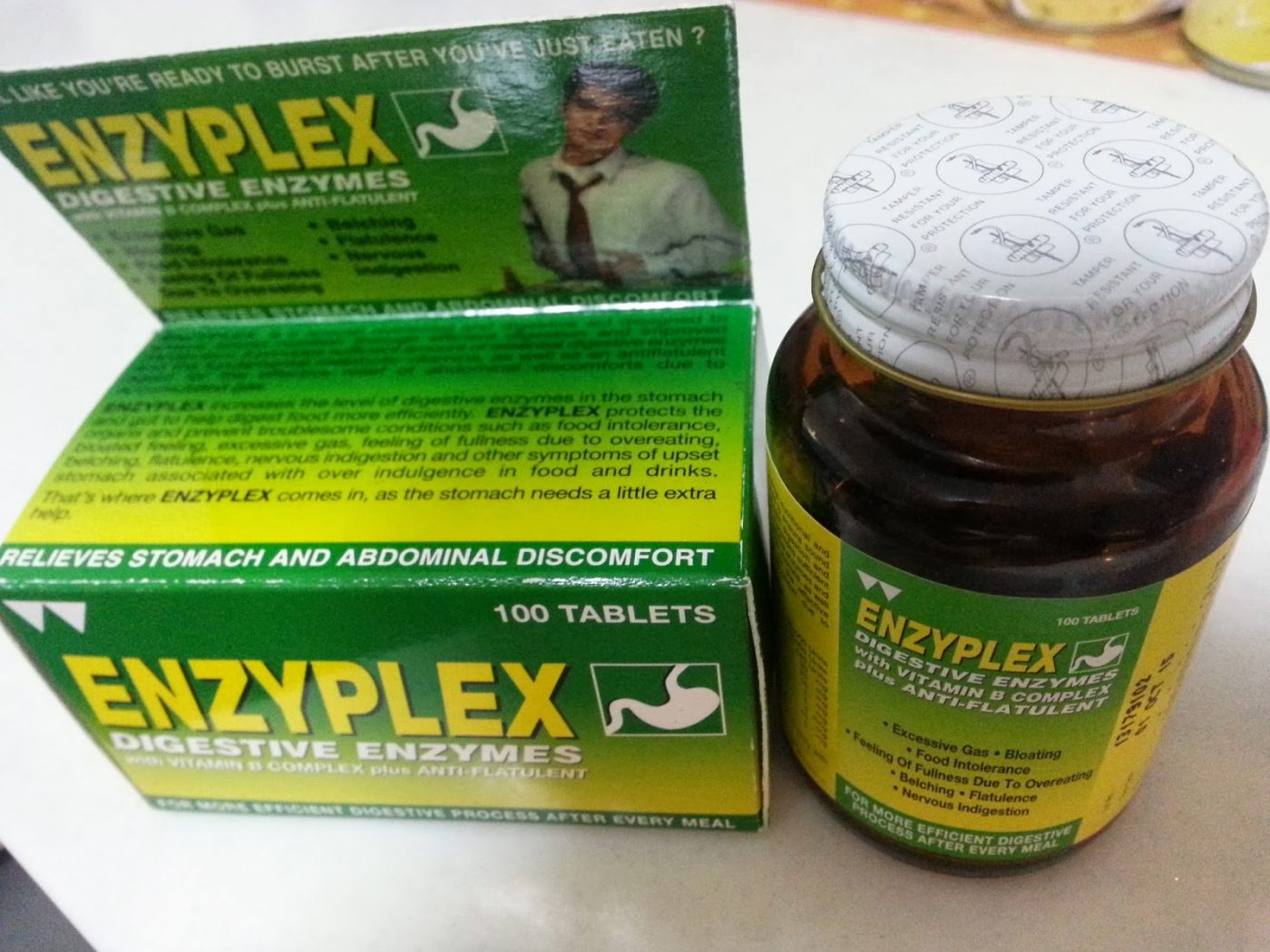
The Health Bureau (SSM) has temporarily banned the over-the-counter medicine Enzyplex, following a contamination scare in Hong Kong.
In a statement on Friday, the Health Bureau said that samples of different batches of the digestive remedy had already been tested, but that it would take five days to get the results. The statement noted that the medicine had been distributed to private hospitals, clinics, and pharmacies in Macau, but that for the time being all prescriptions and sales of the medicine have been suspended.
According to the Macau Post Daily, the SSM statement did not say how many patients who have been prescribed Enzyplex.
According to an RTHK report on Saturday, a type of mould called monascus was earlier found on Enzyplex tablets that were prescribed to a cancer patient at Queen Mary Hospital in Hong Kong who subsequently died. Hong Kong’s Hospital Authority is holding an investigation into the incident and has stopped giving Enzyplex to the 4,000 public hospital patients who previously had been prescribed it.
The RTHK report underlined that according to doctors of the deceased patient her death was not due to Enzyplex, which was manufactured in Indonesia.