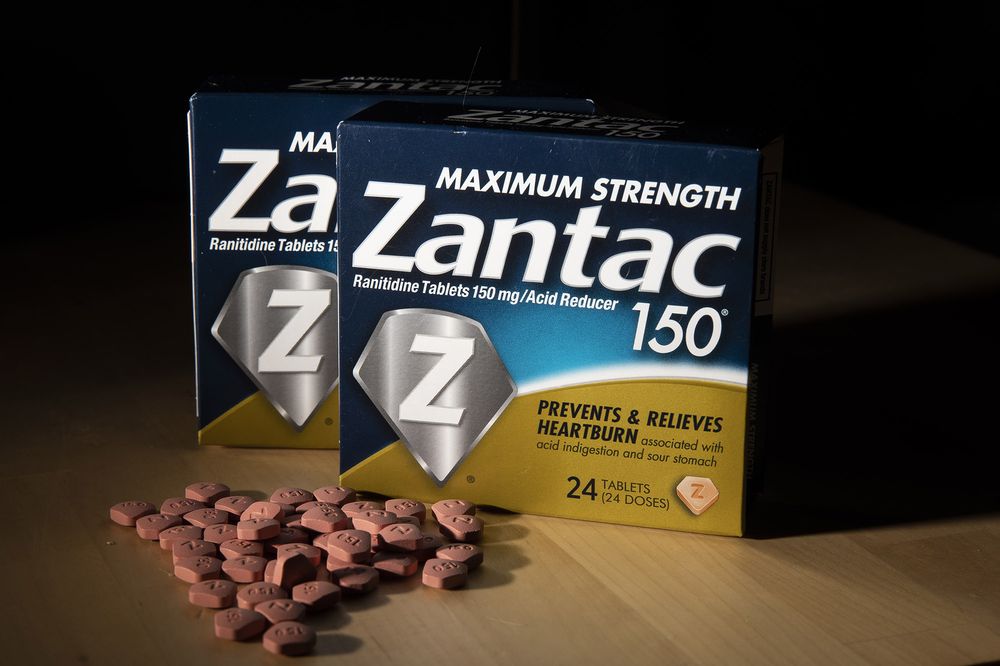
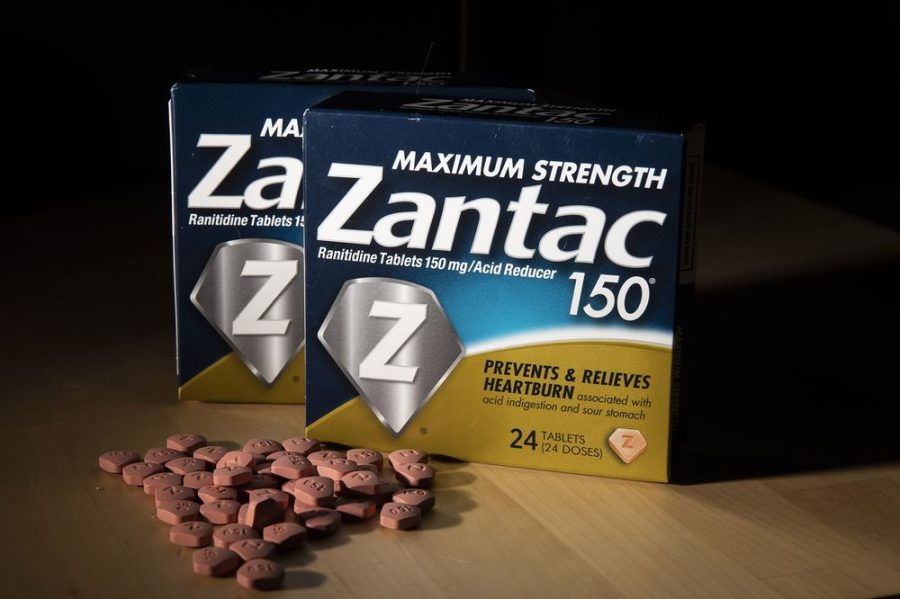
London-based multinational pharmaceutical company GSK has informed a local importer of medicines that it has decided to withdraw four of its drugs sold under the brand name Zantac from the local market, the Health Bureau (SSM) said in a statement on Tuesday.
According to the statement, the four drugs to be withdrawn from the market are Zantak Tablet 150mg, Zantac Tablet 300mg, Zantac Syrup 150mg/10ml, and Zantac Injection 25mg/ml.
According to recent international newswire reports, Zantac, which is made with the raw material “Ranitidine”, is a substance used for the treatment of stomach acidity and related discomforts. The US Food and Drug Administration (FDA) is reported to have recently warned that the medicine could cause cancer due to its possible carcinogenic impurity.
The SSM statement said that a “small quantity” of nitrosamine impurity called N-Nitrosodimethylamine (NDMA) has been found in the four products sold under the trade name Zantac. According to previous international media reports, NDMA is toxic to the liver and other organs and is a probable human carcinogen.
The SSM also said that GSK had informed the local importer that it would suspend the supply of the four Zantac medicines to Macau and request local hospitals, clinics and pharmacies to stop supplying them to patients.
According to the Macau Post Daily, the Health Bureau said in the statement that it had collected Zantac samples for laboratory analysis, promising that the results would be announced as soon as possible. The statement described the withdrawal of the Zantac medicines from the local market as a preventive measure, adding that there existed no evidence that the quantity of NDMA detected in Zantac was posing a health risk to humans.
According to the international newswire reports, a substance that could cause cancer has been found in some Ranitidine heartburn and ulcer medicines, including the brand-name drug Zantac. According to the FDA, the source of the contamination was being investigated, the reports said.